Used to Describe Valence Electron Configuration
The mass of an electron is 11837 of a proton. The octet rule is a chemical rule that generalizes that atoms of low atomic number 20 will combine in a way that results in their having 8 electrons in their valence shells.
The negative charge is equal to 1602 10-19 coulomb in magnitude.
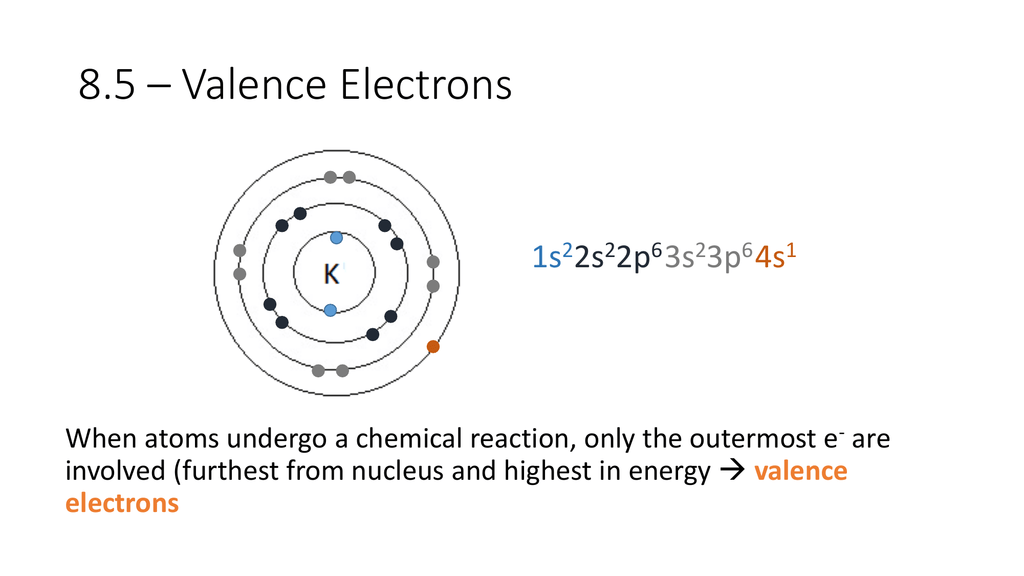
. An electron is a negatively charged particle. Having 8 valence electrons is favorable for stability and is similar to the electron configuration of. The mass of an electron is 910938356 10-31 kilograms.
The mass of the electron is negligible compared to the mass of the proton.

Solved How Do We Define The Valence Electrons For The Main Chegg Com

Valence Electron And Electric Conductivity Electrical4u

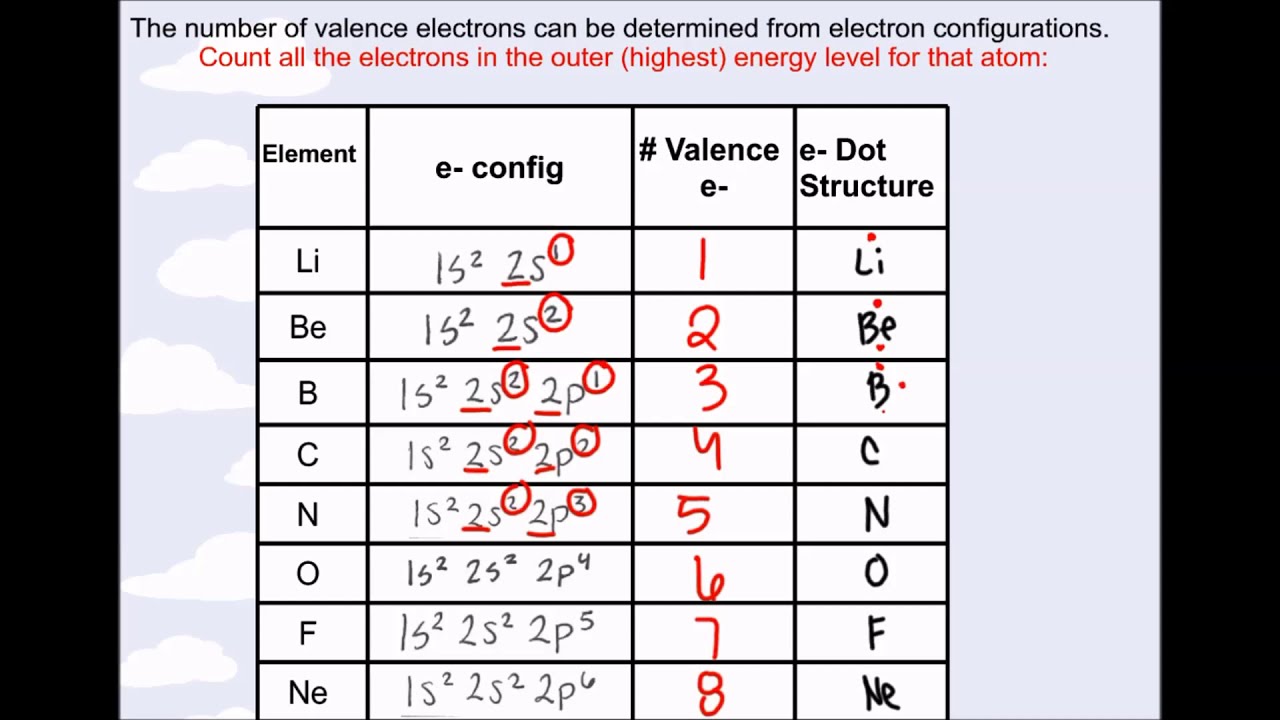
No comments for "Used to Describe Valence Electron Configuration"
Post a Comment